|
Sylvania SHP-TS Mercury-Free |

Recently it has become desirable to eliminate mercury for environmental reasons. Also with normal sodium loss during life, the discharge in the increasingly Hg-rich plasma causes a lamp voltage rise, which leads to cycling and eventual failure.
The development of the high Xenon pressure lamp in the 1980s made it possible to replace the Hg content with a similar pressure of xenon. However since mercury was used to decrease the electrical conductivity of the plasma, its removal means that longer and thinner arc tubes are needed to control the volt drop, such that standard lamps can be retrofitted. This results in a small loss of efficacy compared to "Super" HPS lamps with mercury, but the Hg-free lamps remain more efficient than conventional standard HPS types.
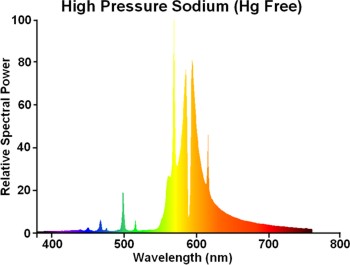
The mercury in standard lamps causes some broadening of the red-wing of the sodium spectrum, and this is absent for the Hg-free lamp (see spectrum below). Consequently the they appear slightly yellower than standard types. The colour temperature is around 100K higher with CRI a little lower, and colour is shifted somewhat away from the blackbody locus.



| Manufacturer: | Sylvania Lighting International | |
| Lamp Power: | 100 Watts | |
| Lamp Current: | 1.2 Amps | |
| Lamp Voltage: | 100 Volts | |
| Cap Type: | E40s/45 | Ni plated brass + vitrite |
| Bulb Finish: | Clear | Soda-lime glass |
| Bulb Type: | T-46 | T-14½ in eighths/inch |
| Overall Length: | 211 mm | 8 5/16 inches |
| Light Centre Length: | 127 mm | 5 inches |
| Arc Length: | ||
| Electrodes: | Backwound Tungsten | BaSr3Y8O16 emitter |
| Atmosphere: | Na,Hg | Xe 300 torr | Outer: Hard Vacuum |
| Luminous Flux: | 10,000 lm | @ 100 hours |
| Luminous Efficacy: | 100.0 lm/W | @ 100 hours |
| Colour Temperature & CRI: | CCT: 2100K | CRI: Ra 18 |
| Chromaticity Co-ordinates: | CCx: 0.527 | CCy: 0.434 |
| Burning Position: | Universal | |
| Rated Lifetime: | 24,000 hours | to 50% survival |
| Warm-up & Re-strike Time: | 3 minutes | 5 minutes |
| Factory: | Tienen | Belgium |
| Date of Manufacture: | March 1996 | Date Code 3 |
| Original & Present Value: | ||